If a reaction only involves a single particle splitting up in

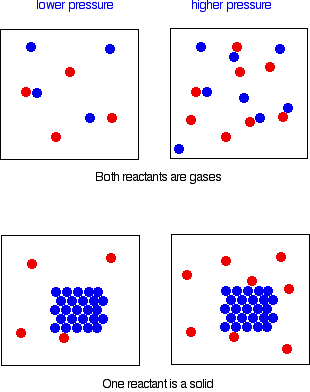
Increasing the surface area of a solid reactant increases the reaction rate.

(b) Stepwise increase in reaction rate as a function of temperature

Factors affecting the rates of Reaction - theory and methods of measuring

The rate of reaction and the chlorine content increases with the temperature

Increasing the temperature increases reaction rates because it increases in

Factors affecting the rates of Reaction - theory and methods of measuring
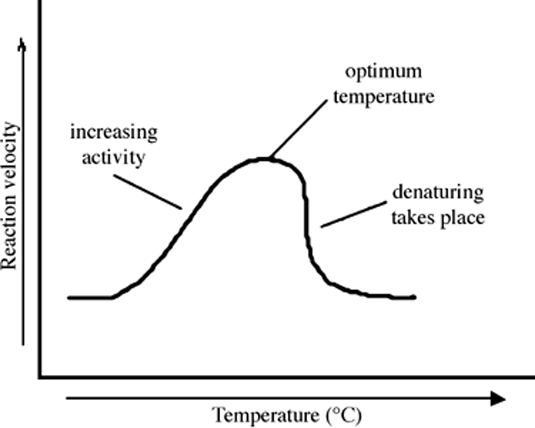
Effect of temperature on reaction rate. The reaction rate increases with
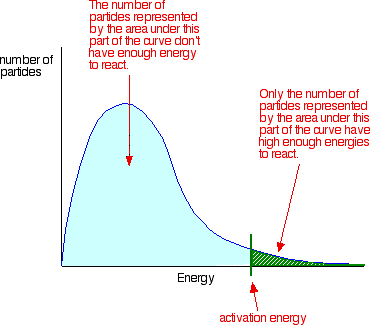
The effect of temperature on rates of reaction

Reaction temperature is a very important parameter which controls the

H2 production rates at the intermediate reaction temperature of 650°C.

1 Denitrification rate to Reaction temperature
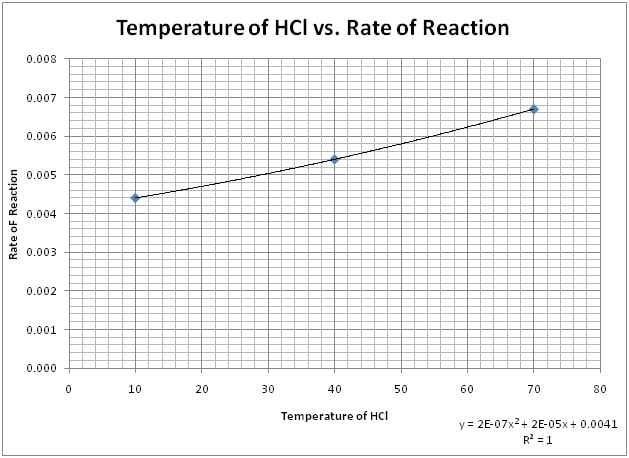
Graph 1: Temperature of HCl vs. Rate of Reaction

free Hantzsch reaction at room temperature with enhanced reaction rates.
The formula that describes the temperature dependence of the rate constant

Temperature vs. rate of reaction. As the temperature increases the particles

1 Denitrification rate to Reaction temperature

To begin with; when temperature is increased the rate of reaction goes up

5: Effect of enzyme concentration on reaction rate T = 21 °C cS = 1.77

ionic strength and temperature, the rate of reaction increased with
